invitro | 인증표준물질(CRM)을 이용한 방사면역측정법의 회수율 평가
페이지 정보
작성자 : 서울대 핵의학과 작성일2017-02-15 조회5,001회관련링크
본문
서울대학교병원 핵의학과
최성희, 신선영, 임소희, 홍미경, 노경운, 김진의
The Evaluation of Recovery Rate of Radioimmunoassay Using Certified Reference Material (CRM)
Sung Hee Choi, Sun Young Shin, So Hee Lim, Mee Kyung Hong, Gyeong Woon Noh and Jin Eui Kim
Department of Nuclear Medicine, Seoul National University Hospital, Seoul, Korea
Purpose: Reference material (RM) is defined as material that is safe and homogeneous enough about specified characteristic that is made with a purpose of using test of measurement or nominal characteristic. Certified reference material (CRM), which is issued by authorized organization, is defined as reference material that provides characteristic value, link uncertainty and retroactivity. The purpose of this paper was to evaluate recovery of radioimmunoassay by Certified Reference Material enclosed with a certificate and therefore to enhance reliability of test. Materials and Methods: WHO certified reference material is purchased from NIBSC (National Institute for Biological Standard and Control, United Kingdom) and made of 3 levels that are C-1 (low concentration), C-2 (medium concentration) and C-3 (high concentration) and measured for kit at the Seoul National University Hospital. Recovery rate is evaluated after measurement at four different days. Results: Recovery rate results using WHO certified reference material are T4 90%, Ferritin 88%, PSA 94%, Prolactin 99%, AFP 94% and TSH 93%. Conclusion: A procedure that appropriate accuracy, precision, specificity, sensitivity, reproducibility, and validate on the subject of kit for radioimmunoassay is essential. Recovery rate assay as extraction efficiency of analysis process is percent about already measuring results of analysis result after all measuring process. This is very important assessment standards of performance evaluation of immunoassay kit. Recovery rate results of 6 type used WHO CRM are satisfactory to 88~99%. This demonstrates that the radioimmunoassay is a very accurate measurement, which is very effectively utilized in clinical practice. (Korean J Nucl Med Technol 2014;18(1):158-162)
Key Words : CRM, RM, Retroactivity, Uncertainty, Recovery, Accuracy
INTRODUCTION
Reference material is a material of substance one or more of whose property values are sufficiently homogeneous and well established to be used for the calibration of an apparatus, the assessment of a measurement method, or for assigning values to materials.1) Certificated reference material (CRM) is reference material, accompanied by a certificate, one or more of whose property values are certified by a procedure which establishes traceability to an accurate realization of the unit in which the property values are expressed, and for which each certified values is accompanied by an uncertainty at a stated level of confidence.1) Radioimmunoassay is based upon the competition between labeled and unlabeled antigen for specific antibody sites, forming antigen-antibody complexes. This reaction is described by the expression. At equilibrium, the radioactive complex is separated from the radioactive antigen. From the nuclear medical aspect, this is now one of the major fields of “in vitro” assay with radioisotope.2) The in vitro lab of Department of Nuclear Medicine performs tests with radioimmunoassay kits. It is essential to examine in vitro diagnostic kit on a regular basis to assure accuracy of the test. Previously, cross institutional and modality experiments were performed using WHO certificated reference materials to assure accuracy of tests, however this study has focused on evaluating recovery of radioimmunoassay by WHO certificated reference materials. The purpose of this paper was to enhance reliability of in vitro diagnostic kit.
MATERIALS AND METHODS
1. Subjects
Thyroxine (T4), Ferritin, Prostate specific antigen(PSA), Prolactin, Alphafetoprotein (AFP) and Thyroid stimulating hormone (TSH) are the main items inspected at the in vitro lab of Department of Nuclear medicine. In this experiment, a total of 6 items was examined.
2. Manufacturing method of Reference material
1) T4 (IRMM-468)
Each vial contains about 100 mg of the powder. IRMM-468(Fig. 1) dissolves more easily in acidic media. Ten milligram of IRMM-468 can be dissolved in a 50 mL volumetric flask by adding 1.5 mL of 1 mol/L HCl, shaking, adding 20 mL of methanol, homogenizing in an ultra-sonic bath and adding methanol up to a volume of 50 mL. Made 10 mg/50 mL is 100 times dilution and make C-1, C-2 and C-3 concentration which were then measured for kit at the Seoul National University Hospital. We used Human serum albumin instead of distilled water.
2) Ferritin (NIBSC 96/670)
Each ampoule contains 6.3 micrograms Ferritin (Fig. 2). Reconstitute the contents of each ampoule were reconstituted with 1.0ml distilled water. No attempt should be made to weigh out any portion of the freeze-dried material prior to reconstitution. 500 ng/mL Ferritin makes C-1, C-2 and C-3 concentration which were then measured for kit at the Seoul National University Hospital. The reference materials of other items were manufactured using the same method as Ferritin.
3. Method of analysis
1) Assays
T4 and PSA were measured by radioimmunoassay (RIA) using commercial kits (Cisbio Bioassay, France). Samples were analyzed for Ferritin, Prolactin and TSH using immunoradiometric assay (IRMA) using commercial kits (Shin Jin Medics Inc., Korea). AFP was determined by IRMA using commercial kits (Immunotech, Czech).
2) Examination of reproducibility
For accuracy test, low, medium, and high concentrate sam ple were experimented four times repeatedly and calculated mean±standard deviation.3)
3) Recovery rate
Pool serum and specific concentrations of sample were mixed and measured the concentration. Recovery rate was calculated using the below formula.3) Recovery rate (%)=measured concentration/theoretical concentration×100
4. Certificated reference materials
WHO certificated reference materials ware purchased by NIBSC and IRMM were made of 3 levels that are C-1 (low concentration), C-2 (medium concentration) and C-3 (high concentration) (Table 1).
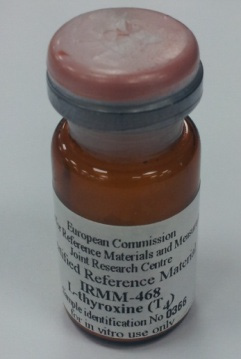
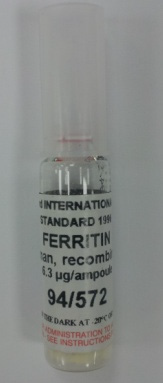
Fig. 1. T4 Reference material (IRMM-468). Fig. 2. Ferritin Reference material (NIBSC 96/670).
Results
The mean value, target value and recovery rate are shown in Table 2. The mean values of C-1, C-2 and C-3 were 7.83, 13.43 and 22.41, respectively. Target values were 8.50, 15.00 and 25.00, respectively, and 92%, 90% and 90% for recovery rate, respectively. Result of Recovery rate using WHO certified reference material was 90% (Table 2). Table 3 shows the results of the mean value, target value and recovery rate of C-1, C-2 and C-3. That were 79.6, 215.8 and 375.9 for mean values and 85.0, 250.0 and 450.0 for target values, respectively, and the recovery rates were 94%, 86% and 84%, respectively. Recovery rate of result using WHO certified reference material was 88% (Table 3). The mean value, target value and recovery rate are presented in Table 4. The results of C-1, C-2 and C-3, respectively were 2.29, 4.19 and 8.79 for mean values and 2.50, 4.50 and 9.00 for target values and recovery rates were 92%. 93% and 98%, respectively. PSA result of recovery rate using WHO certified reference material was 94% (Table 4). Table 5 presents the results of the mean value, target value and recovery rate of C-1, C-2 and C-3. That were 25.6, 49.6 and 85.3 for mean values and 25.00, 50.00 and 90.00 for target values, respectively. The recovery rates of Prolactin were 102%, 99% and 99%, respectively. Recovery rate of result using WHO certified reference material was 99% (Table 5). The mean values of C-1, C-2 and C-3 of AFP were 23.43, 46.78 and 95.92, respectively. Target values were 25.00, 50.00 and 100.00, respectively and recovery rates were 94%, 94% and 96%, respectively. The result rate of using WHO certified reference material was 90% (Table 6). Table 7 shows the results of TSH. The mean values of C-1, C-2 and C-3 were 1.37, 3.79 and 37.75, respectively and 1.50, 4.00 and 40.00 for target values. The recovery rates were 94%, 86% and 84%, respectively. TSH’ result of Recovery rate using WHO certified reference material was 88% (Table 7).
Table 1. Category of WHO certified reference materials
| Items | Institute | Code |
| AFP | NIBSC | AFP |
| Ferritin | NIBSC | 94/572 |
| Prolactin | NIBSC | 84/500 |
| PSA | NIBSC | 96/670 |
| T4 | IRMM | IRMM-468 |
| TSH | NIBSC | 81/565 |
Table 2. Recovery rate of T4 by WHO CRM
| T4 (μg/mL) | 1 | 2 | 3 | Mean |
| 1 | 7.60 | 13.15 | 21.90 | |
| 2 | 7.90 | 13.94 | 22.90 | |
| 3 | 7.95 | 13.15 | 22.85 | |
| 4 | 7.85 | 13.46 | 21.98 | |
| Mean | 7.83 | 13.43 | 22.41 | |
| Target value | 8.50 | 15.00 | 25.00 | |
| Recovery rate | 92% | 90% | 90% | 90% |
Table 3. Recovery rate of Ferritin by WHO CRM
| Ferritin (ng/mL) | 1 | 2 | 3 | Mean |
| 1 | 79.9 | 207.5 | 362.0 | |
| 2 | 78.8 | 215.7 | 397.7 | |
| 3 | 80.1 | 220.4 | 357.9 | |
| 4 | 79.5 | 219.5 | 385.9 | |
| Mean | 79.6 | 215.8 | 375.9 | |
| Target value | 85.0 | 250.0 | 450.0 | |
| Recovery rate | 94% | 86% | 84% | 88% |
Table 4. Recovery rate of PSA by WHO CRM
| PSA (ng/mL) | 1 | 2 | 3 | Mean |
| 1 | 2.26 | 4.37 | 8.52 | |
| 2 | 2.29 | 4.21 | 9.65 | |
| 3 | 2.35 | 4.05 | 8.54 | |
| 4 | 2.25 | 4.14 | 8.44 | |
| Mean | 2.29 | 4.19 | 8.79 | |
| Target value | 2.50 | 4.50 | 9.00 | |
| Recovery rate | 92% | 93% | 98% | 94% |
Table 5. Recovery rate of prolactin by WHO CRM
| PROLACTIN (ng/mL) | 1 | 2 | 3 | Mean |
| 1 | 26.7 | 51.6 | 88.2 | |
| 2 | 27.6 | 49.3 | 86.9 | |
| 3 | 24.1 | 48.8 | 84.5 | |
| 4 | 23.9 | 48.6 | 81.4 | |
| Mean | 25.6 | 49.6 | 85.3 | |
| Target value | 25.00 | 50.00 | 90.00 | |
| Recovery rate | 102% | 99% | 95% | 99% |
Table 6. Recovery rate of AFP by WHO CRM
| AFP (ng/mL) | C-1 | C-2 | C-3 | Mean |
| 1 | 23.71 | 45.63 | 95.52 | |
| 2 | 24.02 | 46.92 | 95.73 | |
| 3 | 22.94 | 47.51 | 96.41 | |
| 4 | 23.05 | 47.05 | 96.01 | |
| Mean | 23.43 | 46.78 | 95.92 | |
| Target value | 25.00 | 50.00 | 100.00 | |
| Recovery rate | 94% | 94% | 96% |
Discussion and Conclusion
Radioimmunoassay has a continuing role in diagnosis, prognosis, treatment and research4) and is a relatively simple, sensitive, and specific method for measuring serum.5) It can report rapidly major experiment that were cancer and hormone experiment for the 2-3 hours.6-7) So Recovery rate experiment of Radioimmunoassay using Certified Reference Material (CRM) is very important. The Certified Reference Material (CRM) plays critical role in experimenting recovery rate of Radioimmunoassay, which have been used to calibrate surface profilers for reliable measurements.8) Organs of supply of Certified Reference Material are NIBSC (National Institute for biological standard and control, United Kingdom) and IRMM (Institute for Reference Materials and Measurements, Belgium). First, NIBSC provides, makes and develops WHO Certified Reference Material of 90% and over. It has several advantages that are high reproducibility, long expiration date. It was simple more than manufacture method of IRMM. IRMM is one of Europe Joint Research Center and global leader in the standardization and control of biological medicines. The in vitro lab of Department of Nuclear Medicine performs examination with radioimmunoassay kits .For accuracy, specificity, sensitivity and reproducibility of Radioimmunoassay kits, appropriate examination of radioimmunoassay kits is essential. The way is examination of recovery rate. For the examination of recovery rate, performed repeated experiment 4 times with certified reference material. Results of recovery rate using WHO Certified reference materials are T4 90%, Ferritin 88%, PSA 94%, Prolactin 99%, AFP 94% and TSH 93%. This demonstrates that the recovery rate went well. To further develop this study, it needs to expand more kits from other laboratories and institutes to increase accuracy and reliability results between each laboratory. It is necessary to continue examination of radioimmunoassay kits with Certified Reference Material.
SUMMARY
공인된 기구에 의해 발급된 문서를 동반하는 인증표준물질은 특성값과 추정값의 신뢰정도를 나타내는 연계 불확도, 그리고 측정한 결과가 명시된 불확정 정도의 범위 내에서 국가 측정 또는 국제측정표준에 일치되도록 연속적으로 비교하고 교정하는 소급성을 제공하는 표준물질이다. 회수율 검사는 검체를 측정하여 얻은 값이 참값에서 얼마만큼 벗어났는지의 차이를 말하며 키트의 정확도를 반영한다. 이러한 인증표 준물질을 가지고 회수율 검사를 하는 것은 체외 방사면역진단 키트의 정확성을 보여주며 이러한 평가는 매우 중요하다. 인
증표준물질은 NIBSC (National Institute for biological standard and control, United Kingdom)와 IRMM (Institute for Reference Materials and Measurements, Belgium)에서 구입하였고 검사 종목은 T4, Ferritin, PSA, Prolactin, AFP 그리고 TSH으로 총 6종목이다. T4는 IRMM의 표준물질을 사용하였고 나머지 종목은 NIBSC의 표준물질을 사용하였다. C-1 (저농도), C-2 (중농도), C-3 (고농도) 3 level로 제조하여 본원에서 사용하는 키트를 이용하여 4회 측정하였다. WHO 인증표준물질을 이용한 회수율 측정에서 T4 90%, Ferritin 88%, PSA 94%, Prolactin 99%, AFP 94%, TSH 93%였다. 6개 종목의 회수율 측정에서 88-99%로 양호한 결과를 보였다.핵의학 체외진단키트의 정확성을 높이기 위해서 이 연구가 다른 검사실의 키트로 확장되어 검사가 되어야 하고 검사실 간에도 교류가 필요하다. 인증표준물질을 이용한 체외진단키트의 회수율 평가가 앞으로도 지속되어야 하며 결과의 정확성은 환자의 만족의 증가로 이어질 것으로 기대한다.
REFERENCES
1. Bernd W. Wenclawiak. Michael Koch . Evsevios Hadjicostas. Quality Assurance in Analytical Chemistry: Training and Teaching. Springer. 1 edition; 2004. p 244-246.
2. Kunio Kurate. The Principle and the Method of the Radioimmunoassy. The Korean Journal of Nuclear Medicine 1970;4:11-18.
3. Au DH, Kim JY, Seok JD. The Usefulness According to the Incubation Time of PTH as Prediction Index of Hypocalcemia. J Nucl Med Technol 2010;14:138-142.
4. Ian B. Hales. The Role of Radioimmunoassay in the Management of Thyroid Disease. Korean Journal of Nuclear Medicine 1984;51-54.
5. Bernard M. Jaffe, Harold R. Behrman. Methods of Hormone Radioimmunoassay. Academic Press. 2nd edition; 1979. p 4-17.
6. Noh GW, Kim TH, Kim JY, Kim HJ, Lee HY, Choi JY, et al. The Evaluation of Proficiency Test between Radioimmunoassay and Chemiluminescence Immunoassay. J Nucl Med Technol 2011;15:116-124.
7. Dong Soo Lee. In Son. Radioimmunoassay. Chang Soon Ko, editors. Korea Medical Publishing Co. 2ndedition; 1997. p803-25.
8. Maeng S, Jin J, Buajarern J, Jae Wan Kim, Kim JA, Kang CS. Design and fabrication of a step height certified reference material for multi-probe inspection instruments. Korea Society of Precision Engineering 2011;28:323-329.